Publications
First authorship is marked in bold. Corresponding author is indicated by *.
Alonso R and Gianoli F, Fabella B, Hudspeth AJ (2025).
Amplification through Local Critical Behavior in the Mammalian Cochlea. Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2503389122Uniquely among our sensory organs, the ear expends energy to amplify the very vibrations that it detects. This “active process” endows the cochlea with exceptional sensitivity, sharp frequency tuning, and broad dynamical range—yet its workings remain elusive. To date, the cochlea’s fragility and inaccessibility have confined studies in vivo, where global phenomena confound local dynamics. Bridging the gap between cellular and whole-organ behavior, we introduce a new preparation that preserves the active process ex vivo in a cochlear segment. We show that the active process operates locally and that the sensory epithelium operates near criticality at a Hopf bifurcation. This result reveals a unified biophysical principle that underlies hearing across insects, nonmammalian vertebrates, and mammals alike.
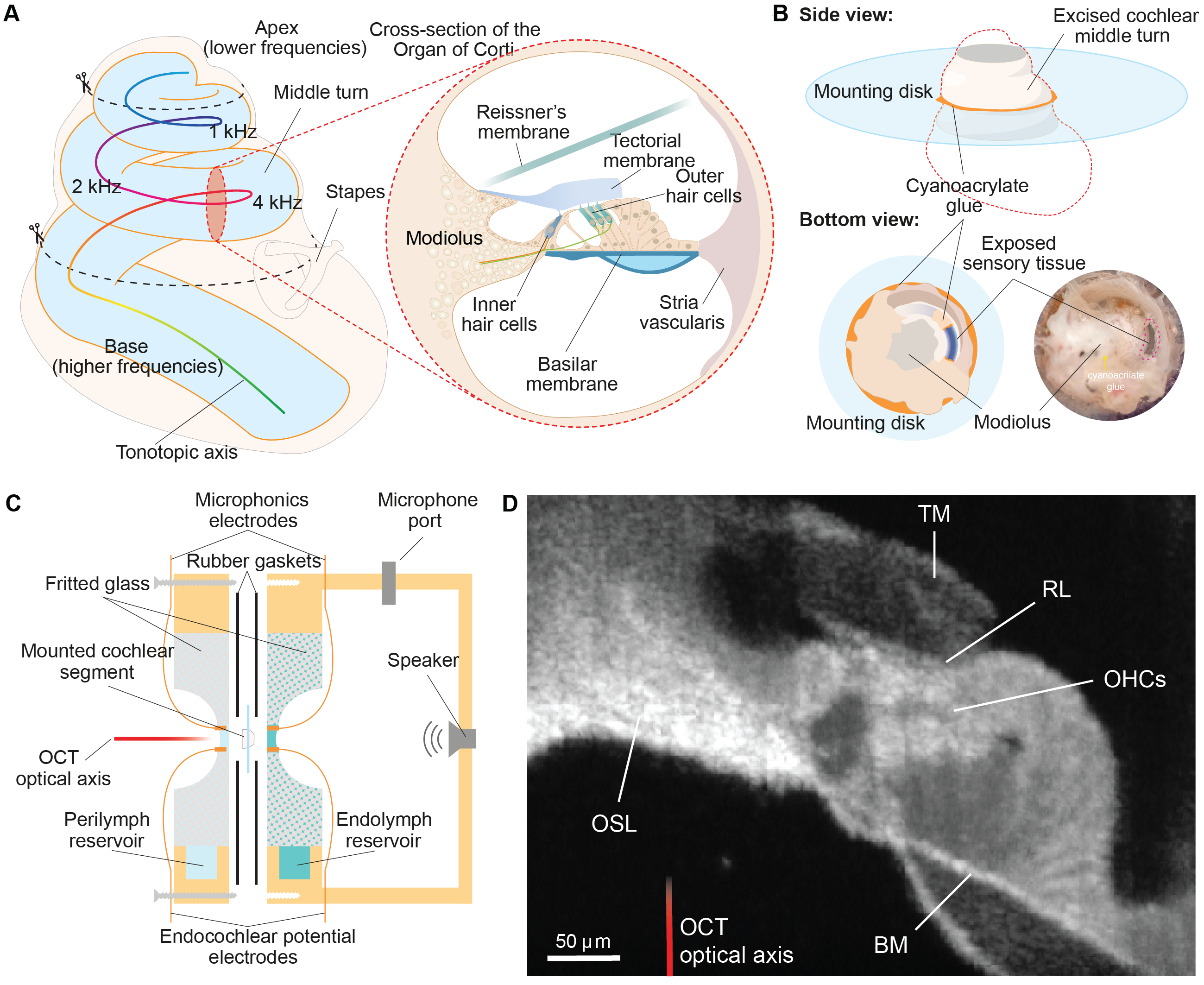
Gianoli F*, Alonso R, Fabella B, Hudspeth AJ (2025).
Toward an ex vivo preparation for studies of the cochlear active process in mammals. Hearing Research.
DOI: 10.1016/j.heares.2025.109288A long-lasting hurdle to the study of the inner workigns of the ear has been the fragility and inaccessibilty of the mammaliann cochlea. In this paper, we developed the LIVE chamber, an custom-made bio-chamber that recreates the physiological environemtn of the inner ear while giving experimental access to the sensory epithelium. Coupled with a novel dissection protocol that preserves the active process ex vivo, this methodology demonstrates that an isolated gerbil cochlear segment can replicate key behaviours observed in healthy ears in vivo. We showed that these observations concide with those expected for a dynamical system operating near a Hopf bifurcation.
Gianoli F*, Alonso R, Fabella B, Hudspeth AJ (2024).
Toward an ex vivo preparation to study the cochlear active process in mammals. Mechanics of Hearing Workshop 2024 (MOH 2024), Ann Arbor, Michigan, USA. Zenodo.
DOI: 10.5281/zenodo.13311903 “Toward an in Vitro Preparation to Study the Cochlear Active Process of Mammals”. Zenodo, August 13, 2024.Peer-reviwed conference paper from the Mechanics of Hearing Workshop 2024 (MOH 2024), Ann Arbor, Michigan, USA.
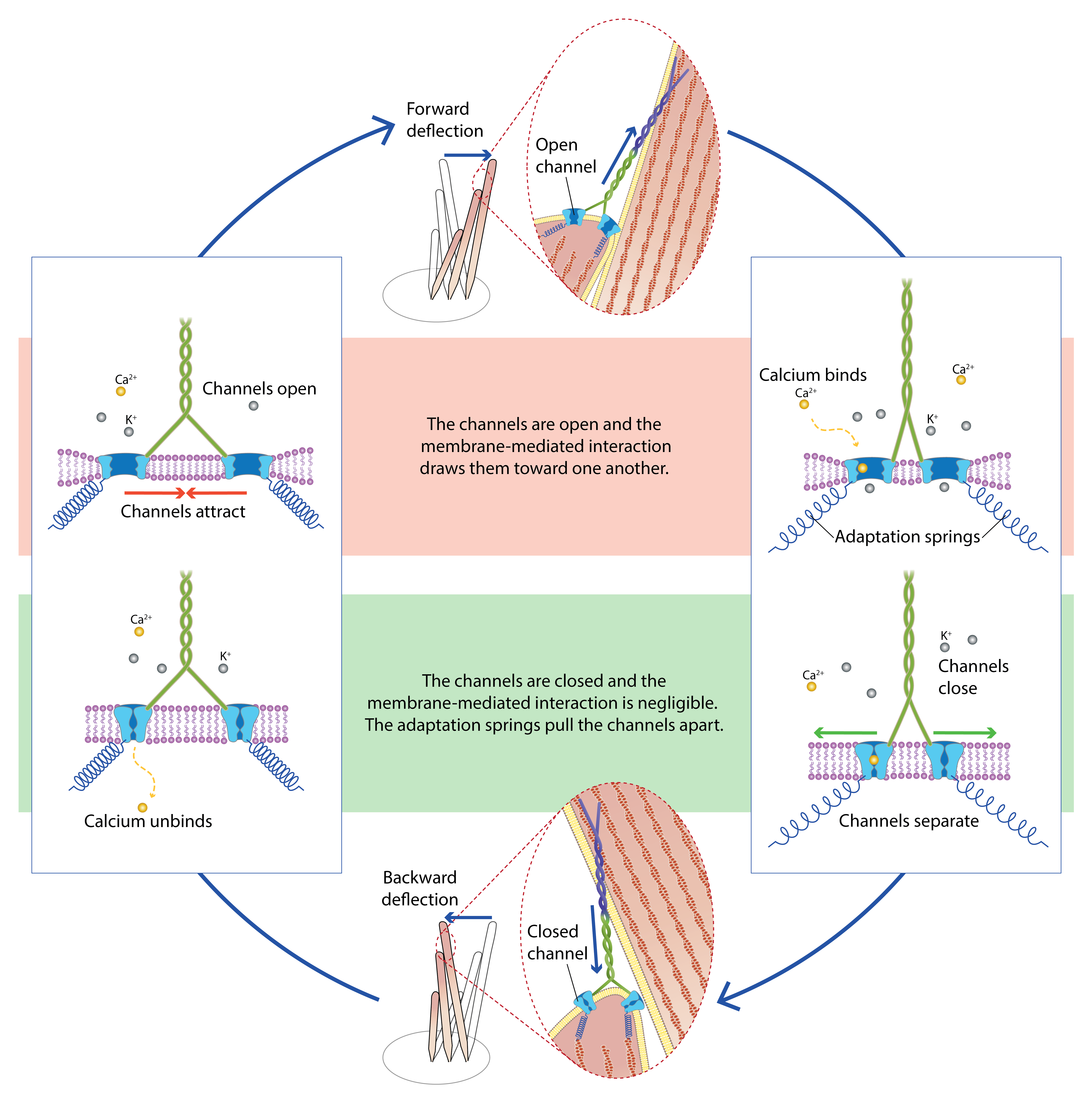
Gianoli F, Hogan B, Dilly É, Risler T, Kozlov AS (2022).
Fast adaptation of cooperative channels engenders Hopf bifurcations in auditory hair cells. Biophysical Journal.
DOI: 10.1016/j.bpj.2022.02.016Building on cooperative-gating model, we incorporated Ca²⁺-binding kinetics to show that hair-cell mechanotransduction channels can spontaneously oscillate via Hopf bifurcations at kilohertz frequencies. The model relies solely on the Ca²⁺ electrochemical gradient as an energy source and membrane-mediated channel interactions to drive this active process. By unifying fast adaptation and channel cooperativity within a single framework, this study provides a mechanistic explanation for high-frequency amplification by hair-bundle motlity in the inner ear.
Abeytunge S and Gianoli F, Hudspeth AJ, Kozlov AS (2021).
Rapid mechanical stimulation of inner-ear hair cells by photonic pressure. eLife.
DOI: 10.7554/eLife.65930The current techniques available to study hair cells, either a piezo-driven glass probe or a fluid jet, are hindered by viscous drag and hence too slow to stimulate hair cells at the physiological timescales of mammalian hearing. The idea behind this paper was to use photonic pressure to circumvent this problem and develop a new method to deliver forces onto hair cells at their physiological frequencies. As we press a letter on a keyboard, the ripple of pressure that spreads through the air peaks at about 20 μPa, a tenth of a billionth of atmospheric pressure—and yet, we hear it. It is not surprising then that the photonic pressure generated by a laser beam could be enough to move the sensitive hair bundles (∼1 pN/nm stiff) of the ear.
Gianoli F, Risler T, Kozlov AS (2019).
The Development of Cooperative Channels Explains the Maturation of Hair Cell’s Mechanotransduction. Biophysical Journal.
DOI: 10.1016/j.bpj.2019.08.042Combining electrophysiological data with a cooperative-gating model, this study shows that the progressive clustering of mechanotransduction channels at tip links during development sharpens mechanosensitivity and narrows the operating range of hair bundles. The model quantitatively reproduces developmental shifts in MET open-probability curves and adaptation kinetics. By revealing channel cooperativity as the driving mechanism behind hair-cell maturation, this work offers a unifying explanation for developmental changes in hearing sensitivity.
Gianoli F (2018).
The GATE-spring theory: a new model of mechanotransduction in auditory hair cells. Doctoral dissertation, Imperial College London.
DOI: 10.25560/78841In this thesis, I extend the classical gating-spring framework by incorporating lipid bilayer effects and paired-channel cooperativity per tip link, resolving structural inconsistencies of earlier models and elucidating the membrane’s role in mechanosensitivity.
Gianoli F, Risler T, Kozlov AS (2017).
Lipid bilayer mediates ion-channel cooperativity in a model of hair-cell mechanotransduction. Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.1713135114This paper focused on developing a new theoretical model of auditory mechanosensitivity in hair cells, addressing a 30-year-old unresolved problem in the classical “gating-spring model” of mechanotransduction, which required an implausibly large structural change of the ion channel upon gating. This model explains various features observed in mammalian hair cells, incorporating the functional role of lipids and cooperative channel gating—now central topics in the field.